ABOUT ANIMAL-FREE CHEMICAL SAFETY ASSESSMENT
About non-animal chemical safety assessment using IATA, DA and NGRA
- What is chemical safety assessment?
- To protect human and environmental health, it is important to ensure that chemicals are used safely This is achieved by global regulations that govern chemical safety such as:
- EU – Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH)
- Canada – New Substances Notification Regulations (Chemicals and Polymers)
- China – Measures for the Environmental Management Registration of New Chemical Substances (Order No. 12)
- Korea – K-REACH
- US – Toxic Substances Control Act (TSCA)
- To protect human and environmental health, it is important to ensure that chemicals are used safely This is achieved by global regulations that govern chemical safety such as:
-
- Chemicals are required by these regulations to go through a set of tests, to provide hazard and risk information for several toxicological endpoints including skin and eye irritation, skin sensitisation, acute toxicity, carcinogenicity, mutagenicity, and reproductive toxicity. Many of these will include animal tests, often producing results not directly linked to, and often irrelevant for, the human outcome.
-
- For regulatory purposes, “chemicals” are defined as those chemical substances with an industrial use, for example, inks, plastics, adhesives/glues, paints, solvents. In this context, the term “chemicals” does not include biocides, pesticides, pharmaceuticals (drugs) or cosmetics (although there is some overlap here as industrial chemicals are often used in cosmetics), as these are usually covered by their own legislation, although specific countries/jurisdictions may vary.
- To address lingering and emerging concerns regarding human and environmental health, there is an urgent need to transition chemical safety testing to modern, species-relevant, New Approach Methodologies (NAMs – non animal approaches that provide information that can be used in hazard and/or risk assessment of chemicals). NAMs, when supported by biological knowledge (e.g. Adverse Outcome Pathways) and used in combination with a decision framework such Integrated Approaches to Testing and Assessment (IATA), Defined Approaches (DA) or Next-Generation Risk Assessment (NGRA) can better protect human health and the environment without the use of animal tests.
- As part of our global capacity-building efforts, we have developed an Education and Training program for NGRA.
- What is a(n):
-
- IATA
- The OECD defines IATA as “pragmatic, science-based approaches for chemical hazard or risk characterisation that rely on an integrated analysis of existing information in a weight of evidence assessment coupled with the generation of new information using testing strategies. IATA follow an iterative approach to answer a defined question in a specific regulatory context, taking into account the acceptable level of uncertainty associated with the decision context”.
- The OECD IATA Case Studies Project was launched in 2015 and allows member countries to gain experience in the use of IATA by sharing and reviewing case studies submitted to the project. The case studies are varied and cover many endpoints e.g. dermal exposure, repeated-dose toxicity, systemic toxicity, developmental toxicity. Learnings from each case study are captured and used to improve future submissions and highlight areas for development of guidance.
- DA
- A Defined Approach (DA) can be used as one of the components of an IATA. As stated in the OECD’s Guidance Document On The Reporting Of Defined Approaches To Be Used Within IATA a DA consists of “a fixed data interpretation procedure (DIP) applied to data generated with a defined set of information sources to derive a result that can either be used on its own, or together with other information sources within an IATA, to satisfy a specific regulatory need. Thus, a defined approach to testing and assessment can be used to support the hazard identification.”
- Many DAs for skin sensitisation have been developed and two (the 2o3 and the ITS) have been accepted by the OECD for inclusion in Guideline No. 497 – Defined Approaches for Skin Sensitisation. The 2o3 uses two concordant results from either the DPRA, KeratinoSens™, or the h-CLAT skin sensitisation assays to predict hazard (sensitiser/non-sensitiser). The ITS applies a score (between 0-3 for each) to results from the DPRA, the h-CLAT and an in silico model (v1 uses Derek Nexus, v2 uses OECD QSAR Toolbox) to predict UN GHS sub-categories (1A/1B/NC).
- NGRA
- Next Generation Risk Assessment (NGRA) is defined as an exposure-led, hypothesis-driven risk assessment approach that integrates all types of information, relying prominently on NAMs in an iterative process designed specifically for the substance and the question being asked.
- NGRA is based on nine principles that were developed by the International Cooperation on Cosmetics Regulation (ICCR)
- The overall goal is a human safety risk assessment
- The assessment is exposure-led
- The assessment is hypothesis-driven
- The assessment is designed to prevent harm
- The assessment follows an appropriate appraisal of all existing information
- The assessment uses a tiered and iterative approach
- The assessment uses robust and relevant methods and strategies
- Sources of uncertainty should be characterized and documented
- The logic of the approach should be transparent and documented
- AOP
-
- An Adverse Outcome Pathway (AOP) describes the events that occur following chemical exposure, beginning with the molecular interaction of the chemical with a biomolecule (e.g., a protein, receptor, etc.) – the molecular initiating event (MIE) – followed by a description of the sequential cellular and tissue perturbations known as Key Events (KE) that lead to eventual toxicological effect – or adverse outcome (AO) (Figure 1). The KE linked to the AO are measurable and as such, the quantitative information included in the relationships between KE (KER), can be very useful within a predictive toxicological framework like an DA, an IATA or an NGRA.
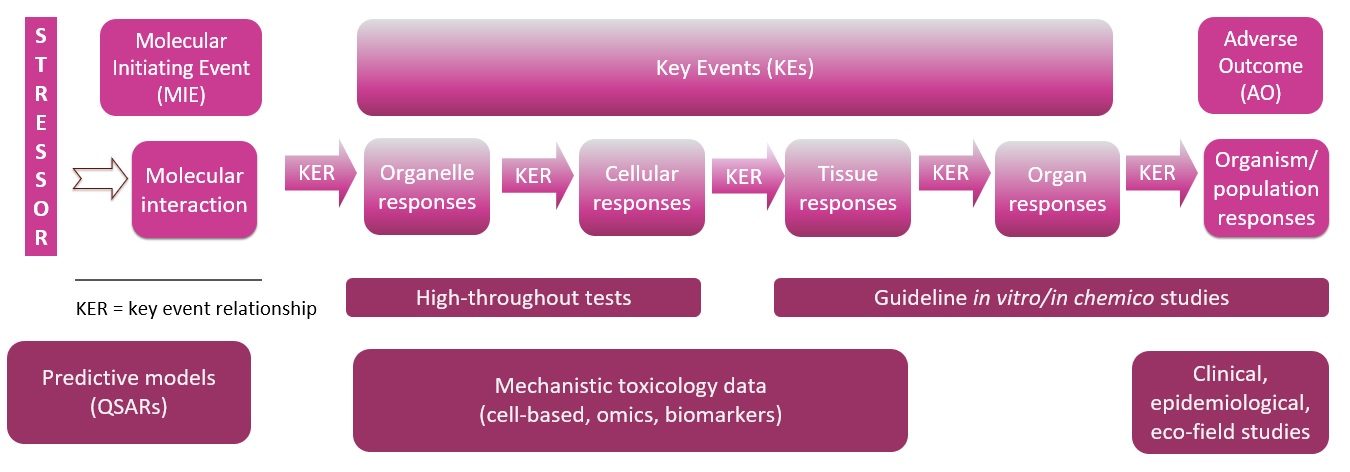
Figure 1. Diagram of an AOP.
To learn more about AOPs and for training resources please click here.
- IATA
- How are IATA, NGRA and DA used in hazard and risk assessment?
-
- The IATA and NGRA frameworks are flexible and allow streamlining of the testing and assessment process to generate exactly the information needed to make a decision. IATA and NGRA can be as simple or as complex as needed to answer the specific question, depending on the amount of certainty needed. They both begin with the problem formulation – what is the question that needs to be answered and what is the context of that question?
- Next, potential use and exposure to the chemical is considered. Then all existing information is gathered, a weight of evidence consideration applied to that information, and then the question is re-assessed to see if it has been answered with sufficient certainty. If not, additional information may be generated.
-
- Several NGRA case studies have been published.: