The ATT and the TABST are old safety test used respectively in human and veterinary vaccine production, the roots of which can be traced back to the beginning of the 20th century to testing for phenol preservatives. The test has no analytical component: it is a pass/fail quality procedure in which mice or guinea pigs are injected many times the human dose of vaccine, and then followed to see whether abnormal reactions are observed. In the absence of alternative strategies to ensure safety, ATT and TABST have become in the course of an entire century de facto standards, required for decades by every pharmacopoeia in the world, a role they have maintained even in light of ever mounting evidence of their poor scientific foundation, and later confirmation of their demonstrated irrelevance. The WHO’s Expert Committee on Biological Standardization itself weighted in the matter, issuing in 2018 its recommendation that ATT be ignored whenever mentioned in WHO documents (Lei D, et al., Removal of the innocuity test from The International Pharmacopoeia and WHO recommendations for vaccines and biological products. Biologicals. 2020 Jul; doi: 10.1016/j.biologicals.2020.05.003.).
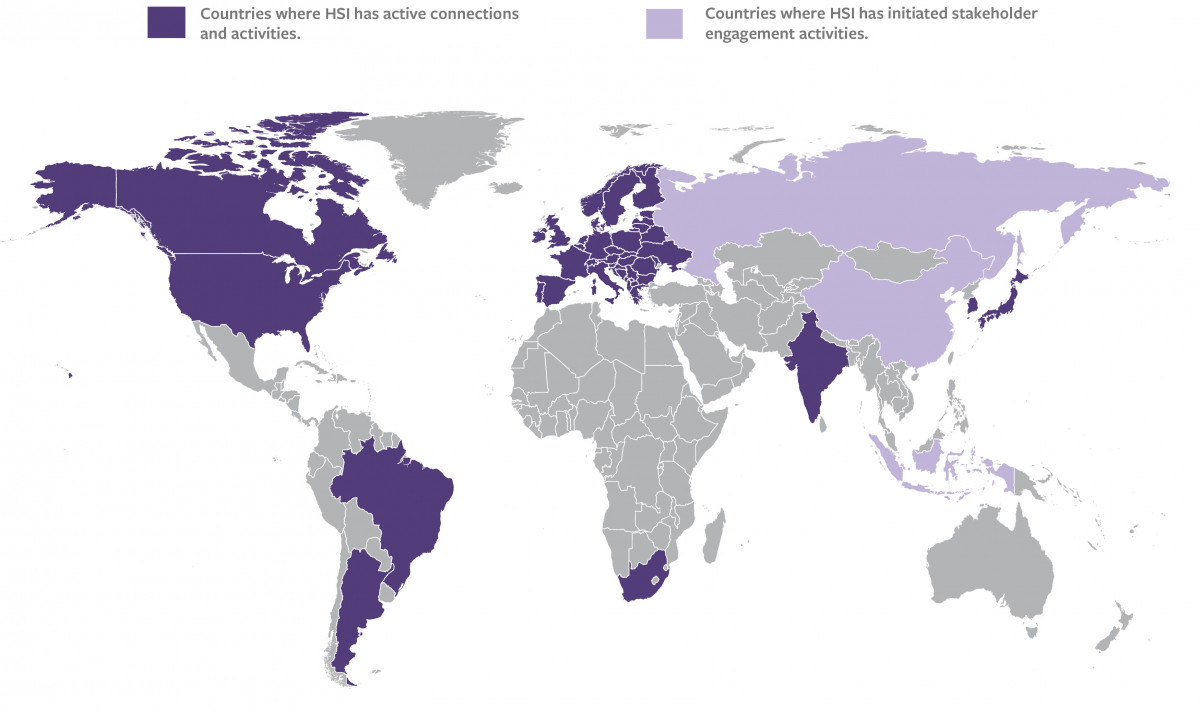
A number of pharmacopoeias have finally proceeded to ATT deletion (e.g. Europe, USA, Canada, Japan, Brazil, Argentina, and partially India – see the full list in the table below), and almost every country in the world have either deleted or waived TABST, with some important exceptions. A number of countries are considering granting waivers to their use, and/or deletion, supported by manufacturers, for which ATT and TABST represent extra costs, extra risks (due to their inherent variability which can make safe batches fail the test), and are not applicable to newer vaccines designed from the start for different, non-animal based safety testing strategies.
For the many countries where ATT and TABTS are still considered irreplaceable tests and ultimate guarantors of vaccines safety, their elimination requires appropriate engagement of local authorities, with exchange of information on the successful deletions already achieved in other countries/regions, and trust building. The discussion needs to be prompted and fostered, consider barriers and difficulties still hindering the elimination of the tests, enriched by the participation of key international stakeholders and experts which can contribute experiences and expertise, and help resolve doubts and assuage fears, to help regulators embrace change and produce actionable items to bring forward the elimination of the tests.
AFSA endeavours have produced a number of successes and achievements:
- Complete and partial deletion of the Abnormal Toxicity Test for human vaccines in:
- Brazil (2019); India (2021); South Korea (2022); Indonesia (2022; product specific waivers after company’s request)
- Russia (eased position toward test waiver, deletion and reduction, and established channels for communications and exchange till February 2022)
- Implemented regulatory requirement for the waiver of the Animal Batch Safety Tests (Veterinary vaccines):
- Brazil (2022)
- India (2022; eased position toward test waiver; ongoing cooperation)
- Organized dedicated local and global multi-stakeholders’ meetings and workshops:
- Rome (2019): “Global harmonization of vaccine testing requirements: making elimination of the ATT and TABST a concrete global achievement” (Biologicals 2020; AFSA Roadmap).
- Bangkok (2019): Support in the organization of the IABS Conference “Animal testing for vaccines. Implementing replacement, reduction and refinement: challenges and priorities” (Biologicals, 2020).
- Joint AFSA-EFPIA Workshop (2021, online workshop): The workshop aimed to assess the lingering barriers, to reach a shared agreement on further concrete actions to make the deletion of the test a global concrete achievement. International organisations and funding bodies, industry and its associations, and regulatory stakeholders were invited to share their view and discuss the respective experiences in a multi-stakeholder environment (Biologicals, 2022) (video)
- India (2021, online workshop): “Future of TABST and LABST in the Indian Pharmacopoeia Monographs. A Humane Society International/India Workshop” (manuscript under review in Biologicals). The workshop was attended by 20 local and international stakeholders from the Indian Pharmacopoeia Commission (IPC), the Central Drugs Standard Control Organisation (CDSCO), industry representatives from the Indian Federation for Animal Health Companies (INFAH), the Asian Animal Health Association (AAHA) and HealthforAnimals, and from EDQM and VICH. (Biologicals, 2023)
AFSA’s Objectives and Roadmap
Wanting to be part of this momentous change, AFSA is helping involved stakeholders exchange information, experience, training and materials, and assisting them in their move away from animal methods.
Download the AFSA Roadmap for the Elimination of ATT, TABST & LABST

Workshop Documents
Keynote Presentations:
- The Global Challenge of Post Approval Changes (PACs)
- The European Directorate for the Quality of Medicines & Healthcare (EDQM)
Read the Workshop report in Biologicals: Accelerating Global Deletion of the Abnormal Toxicity Test for vaccines and biologicals. Planning common next steps. A workshop Report, Biologicals, 2022.
Recording
ATT Global Requirements
| Country | Status | Reference |
| USA | Deleted (2015) | Revocation of General Safety Test Regulations That Are Duplicative of Requirements in Biologics License Applications (link) |
| Canada | No longer required (2007) | As of 2007, requests to delete the General Safety Test (GST) for approved vaccines were accepted, and the GST was not required for new vaccine authorizations. |
| Europe | Fully Deleted (2019) | 1998 – Deleted for batch release from > 80 other monographs; complete removal from monographs for veterinary medicines, human sera and immunoglobulins, diphtheria, tetanus and pertussis vaccines and moved to the production section for other relevant human medicines. Decision taken by the European Pharmacopoeia Commission during the 159th plenary session (Strasbourg on 21-22 November 2017) to delete all remaining ATT entered into force on January 1st, 2019 (link) |
| Argentina | Partially Deleted (2018-) | Deleted from all human vaccines. Deletion activities ongoing for other biologicals products. Revision of the Argentinian Pharmacopoeia (7th Edition, 2003) ongoing. Volume 1. Chapter: 360. ENSAYO DE TOXICIDAD ANORMAL |
| Brazil | Partially deleted (2019- ) | Brazilian Pharmacopoeia 6th Edition (only one exception: required for monograph “Meglumine antimoniate, injectable solution”) |
| India | Deleted from most of the specific monographs of human vaccines (2020- ) | IP 2018, Amendment List 06 of July 22nd, 2020 (link) delete ATT for all listed products (human vaccines). In IP 2022, ATT requirement remains for new human vaccines and for 12 other products (Sterile bags; Chorionic gonadotrophin injection; Menotropin; Protamine sulphate injection; Streptokinase; Streptokinase injection; Urokinase; dried human anti-haemophilic fraction; human albumin; human normal immunoglobulin; human plasma protein fraction; recombinant streptokinase injection). |
| South Africa | Not performed (end of 2018) | Viviani et. al (2020) |
| Russia | Required (waiver possibilities) | Russian Pharmacopoeia Edition XIV. OFS:1.2.4.0004.15. Аномальная токсичность. Государственная фармакопея Российской Федерации. XIII изд. Т. 1. М.; 2015. Government Decree No. 1510 (2019) ‘On the Procedure of Batch Release of Medicinal Products for Human Use’ |
| China | Required (waiver possibilities) | Chinese Pharmacopoeia 2020. Chapter 1141. 异常毒性检查法 |
| Japan | Partially waived (2020) | Amendment Act (2020) of the Minimum Requirements for Biological Products (link). Waiver from some products: Influenza HA vaccine, Japanese encephalitis vaccine, Haemophilus influenzae type B (Hib), pneumococcal polysaccharide vaccine (PPSV23). April 28th 2022: ATT are no longer needed to ensure the safety of vaccines, including recombinant VSV, HPV and HBV vaccines. |
| South Korea | Deleted (2022) | Amendment to the Regulation on Approval and Review of Biologicals Products (link) |
| Indonesia | Required (waiver possibilities) | Indonesian Pharmacopoeia 6th Edition (2020). Vaksin, page 64: “Toksisitas Abnormal Memenuhi syarat Uji toksisitas abnormal seperti yang tertera pada Uji Reaktivitas secara Biologis in-vivo <251>, kecuali dinyatakan lain dalam monografi.” Product specific waivers have been granted to manufacturers (2022) |
| Thailand | Deleted | WHO 69th Expert Committee on Biological Standardization held from 29 October to 2 November 2018 (link). Manufacturers report that ATT is still requested but waivers are granted depending on the type of products (vaccine vs antivenom). |
| Taiwan | Required | |
| Vietnam | Required | |
| Mexico | Required (waiver possibilities) | Review of the pharmacopeia for ATT deletion planned for 2023. |
| Chile | Required | |
| Malaysia | Required | Not performed, manufacturers’ request for waivers pending authorities’ decision |
| Pakistan | Required | Not performed, manufacturers’ request for waivers pending authorities’ decision |
| Egypt | Required | Not performed, manufacturers’ request for waivers pending authorities’ decision |
| Turkey | Required (waiver possibilities) | Manufacturers reported acceptance of waiver request |
| Saudi Arabia | Required | |
| Laos | Required | |
| Cambodia | Required | |
| Myanmar | Required | Manufacturers’ request for waivers pending authorities’ decision |
| Philippines | Required | Manufacturers’ request for waivers pending authorities’ decision |
| GCC – Cooperation Council for the Arab States of the Gulf | Required | Manufacturers’ request for waivers pending authorities’ decision |
| Iran | Required | Manufacturers’ request for waivers pending authorities’ decision |
| Kenya | Required | Manufacturers reported acceptance of waiver request |
| Zambia | Required | Manufacturers reported acceptance of waiver request |
| Ghana | Required | |
| Nigeria | Required | |
| Zimbabwe | Required | |
| Nepal | Required | Manufacturers reported acceptance of waiver request |
| Uganda | Required | |
| Uzbekistan | Required | |
| Ukraine | Required |