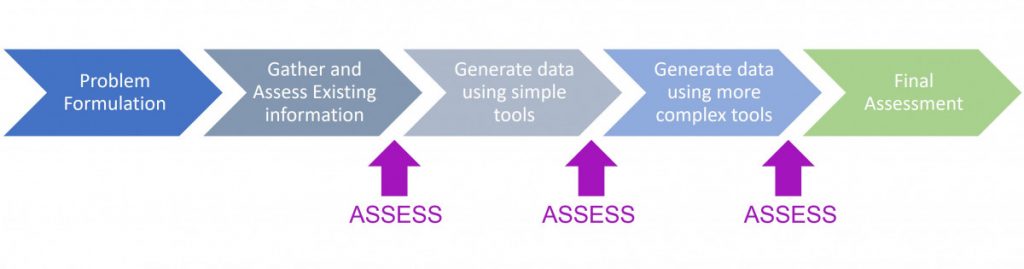
The AFSA E&T course describes a flexible risk assessment approach that begins with problem formulation (a description of the decision context: e.g. what is the chemical, product, use and biological activity being addressed?), followed by gathering and evaluating all of the existing information as well as any exposure and/or bioactivity modeling that can readily be done. If a decision can be made at this stage, the assessment is done. If more information is needed, an iterative information gathering process is carried out, with the most straightforward tests and predictive models employed first, with more complex and resource-intensive methods employed in subsequent tiers as needed to reach a decision with the necessary certainty.
Risk Assessment Process
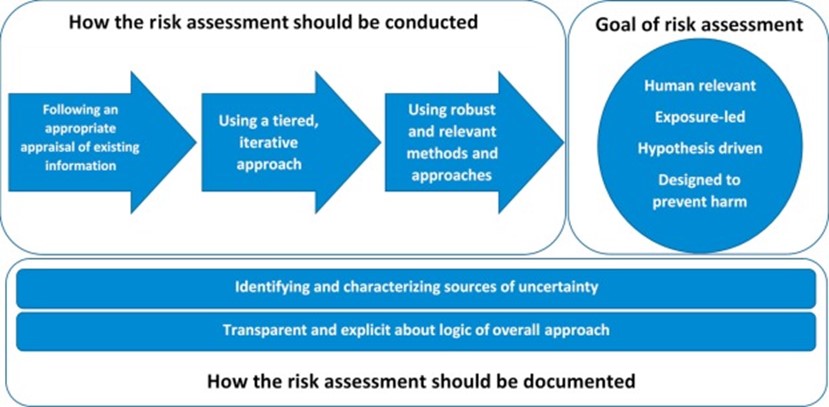
Next Generation Risk Assessment – NGRA – is defined as an exposure-led, hypothesis-driven risk assessment approach focused on human health that integrates all types of information, relying prominently on new approach methods (NAMs – defined as methods that do not involve animals), in an iterative process designed specifically for the substance and the question being asked.
NGRA is based on nine principles that were developed by the International Cooperation on Cosmetics Regulation (ICCR), as well as a tiered workflow that was developed during the European Commission Framework Programme 7 project, Safety Evaluation Ultimately Replacing Animal Testing (SEURAT), and further revised in case studies.
The ICCR is a collaboration of regulators from around the world (including Brazil, Canada, Chinese Taipei, the European Union, Japan, Republic of Korea, and the United States). The ICCR NGRA principles were developed in collaboration with industry partners and cover the design, execution and documentation of the NGRA:
Overarching principles:
- The overall goal is a human safety risk assessment
- The assessment is exposure led
- The assessment is hypothesis driven
- The assessment is designed to prevent harm (i.e. distinguish between adaptation and adversity)
The following three principles describe how a NGRA should be conducted:
- Using a tiered and iterative approach
- Following an appropriate appraisal of existing information
- Using robust and relevant methods and strategies
Finally, two principles for documenting NGRA are described:
- The logic of the approach should be transparently and explicitly documented
- Sources of uncertainty should be characterized and documented
Figure 1: ICCR Principles underpinning the use of new methodologies in the risk assessment of cosmetic ingredients
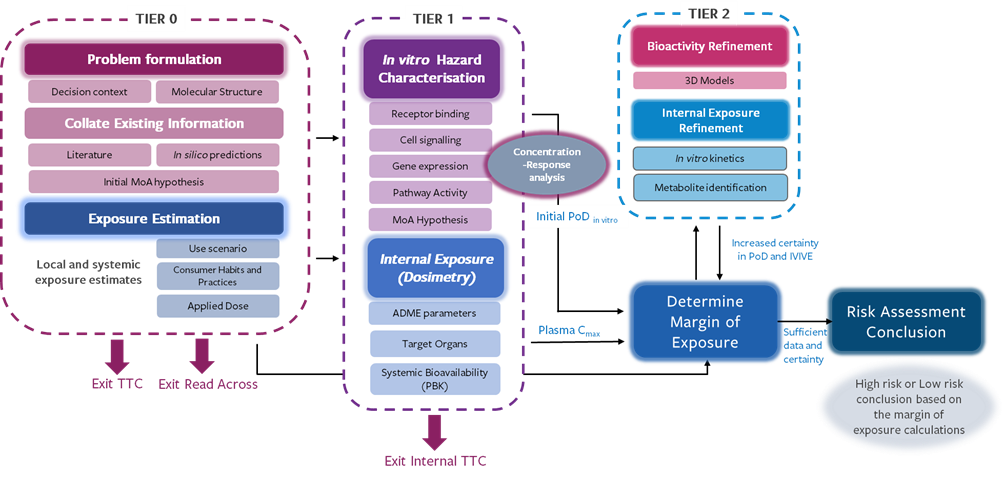
Next Generation Risk Assessment
The process follows a workflow initially described by the European Commission Framework Programme 7 project Safety Evaluation Ultimately Replacing Animal Testing (SEURAT). The workflow consists of three tiers of assessments, each with a possibility for reaching a conclusion and exiting the assessment process.
- Tier 0: Problem formulation, data gathering and initial assessment
Starts with problem formulation, including the chemical identity and structure, the product use scenarios, and the collection and evaluation of all existing data, and if appropriate, the identification of analogues to perform a read-across. It may be possible at this stage to form an initial hypothesis regarding mode or mechanism of action. It may also be possible to reach a safety conclusion based on the toxicological threshold of concern or by read-across. - Tier 1: Hypothesis generation and testing in vitro
Initial in vitro hazard characterization and analysis of internal exposure (systemic bioavailability). It may be possible to reach a conclusion at this point by a consideration of the internal threshold of toxicological concern or via an initial determination of the margin of exposure, which is a comparison of the internal exposure and the concentration of chemical that is predicted to cause a potentially adverse biological activity. - Tier 2: further bespoke in vitro testing as needed
Application of ab initio approach, including tiered targeted testing, refinement of biokinetics, estimation of points of departure in vitro, in vitro to in vivo extrapolation, estimation of uncertainty, and a refined estimation of margin of safety and the final safety assessment based on NAMs (PBK modelling, ‘omics, reporter gene assays, in vitro pharmacological profiling, 3D culture systems, organs‐on‐chip, zebrafish larva assays, pathways modelling, human studies).
Figure 2: NGRA workflow
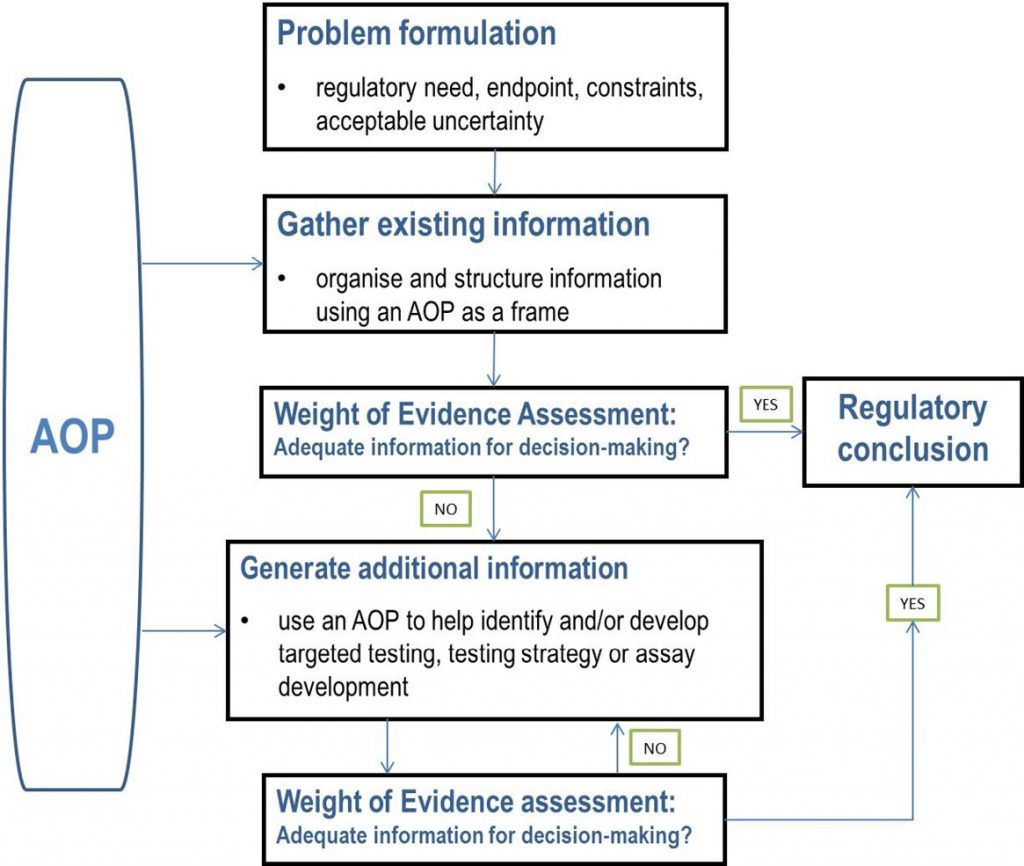
As described by the Organization for Economic Cooperation and Development (OECD), Integrated Approaches to Testing and Assessment (IATA) is a general approach to chemical risk assessment that is conceptually and structurally similar to NGRA in that the process begins with problem formulation and proceeds with an integrative evaluation and data gathering process until there is enough information to come to a conclusion. IATA can also be informed by knowledge from Adverse Outcome Pathways (AOPs).
NGRA could be considered a special case of IATA where the focus is human health, the process is exposure-led, and the information generated is strictly in vitro (or in silico). (See Figure 3 below.) Both the OECD IATA framework and the SEURAT workflow problem formulation can be adapted to address any safety decision context, from internal decision making to regulatory application.
Figure 3: IATA Diagram